Generation and Cultivation of 3D Cell Models
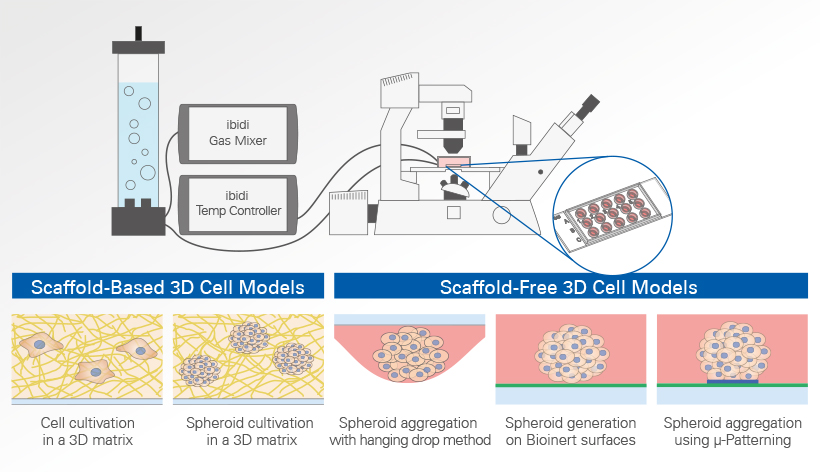
Common methods for the generation and cultivation of 3D cell models include the hanging drop method, µ-Patterning for spheroid aggregation, and the use of scaffolds such as collagen or Matrigel®. Also, cultivation of single cells in an extracellular matrix (ECM) is considered a 3D culture. Each method has its own advantages and disadvantages, which we will look at in more detail here.
- Cell Cultivation in a 3D Matrix
- Spheroid Aggregation With the Hanging Drop Method
- Spheroid Aggregation on Bionert Surfaces
- Spheroid Aggregation Using µ-Patterning
Cell Cultivation in a 3D Matrix
Hydrogel-based scaffolding materials such as collagen or Matrigel® support three-dimensional structures and mimic the extracellular matrix found in tissues. These scaffolds create an architecture that closely resembles the in vivo environment, offering essential physical support and biochemical cues for cell growth and differentiation.
Collagen is the most abundant protein in mammals and used in cell culture to form a hydrogel by reassembling into a fibrous network, which provides a natural environment for cells. Matrigel® is derived from Engelbreth-Holm-Swarm mouse sarcoma and contains ECM proteins like laminin and collagen IV, but also several less defined growth factors. It transitions to a hydrogel at body temperature, creating a 3D environment for cell culture and tissue engineering.
The use of ECM scaffolds is the current standard for the cultivation of organoids. Using hydrogels for 3D cell cultivation offers distinct advantages and disadvantages compared to scaffold-free methods like the hanging drop technique or the µ-Patterning technology. Hydrogels more closely replicate the extracellular matrix (ECM), providing a more physiological environment for cells, which is crucial for studying cell behavior, differentiation, and tissue development. Both, collagen and Matrigel®, can be modified in terms of stiffness and composition to suit different cell types and research needs. However, hydrogels have certain limitations. Unlike µ-Patterning, they do not allow for precise control over the spatial arrangement of cells, which can be crucial for certain applications like tissue engineering. Additionally, natural hydrogels may exhibit a batch-to-batch variability, potentially affecting the reproducibility of results.
3D cell aggregates often more accurately mirror in vivo conditions compared to single-cell cultures. However, it is also possible to culture and image single cells within a 3D gel, offering a unique perspective for various biological studies. This approach is particularly useful for investigating migration kinetics, cell-matrix-interactions and tube formation processes. In addition to cultures with only one cell type, the invasion behavior of two different cell types (e.g., cancer cells and fibroblasts) can be investigated by co-culturing them in the same vessel.
Although this method often does not match the complexity and fidelity of spheroid or organoid culture, it remains a cost-effective and straightforward alternative for research questions specifically centered on single-cell dynamics and responses.
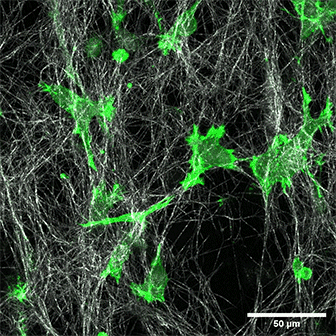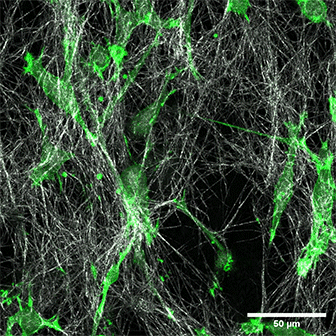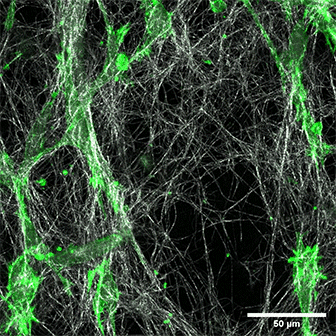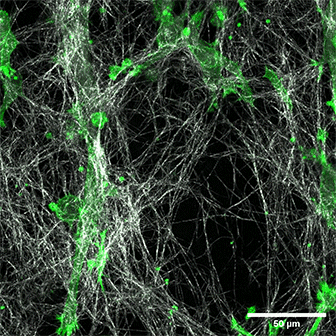
LifeAct-expressing HT-1080 cells (green) in a Collagen Type I, Rat Tail layer in the µ-Slide Chemotaxis.
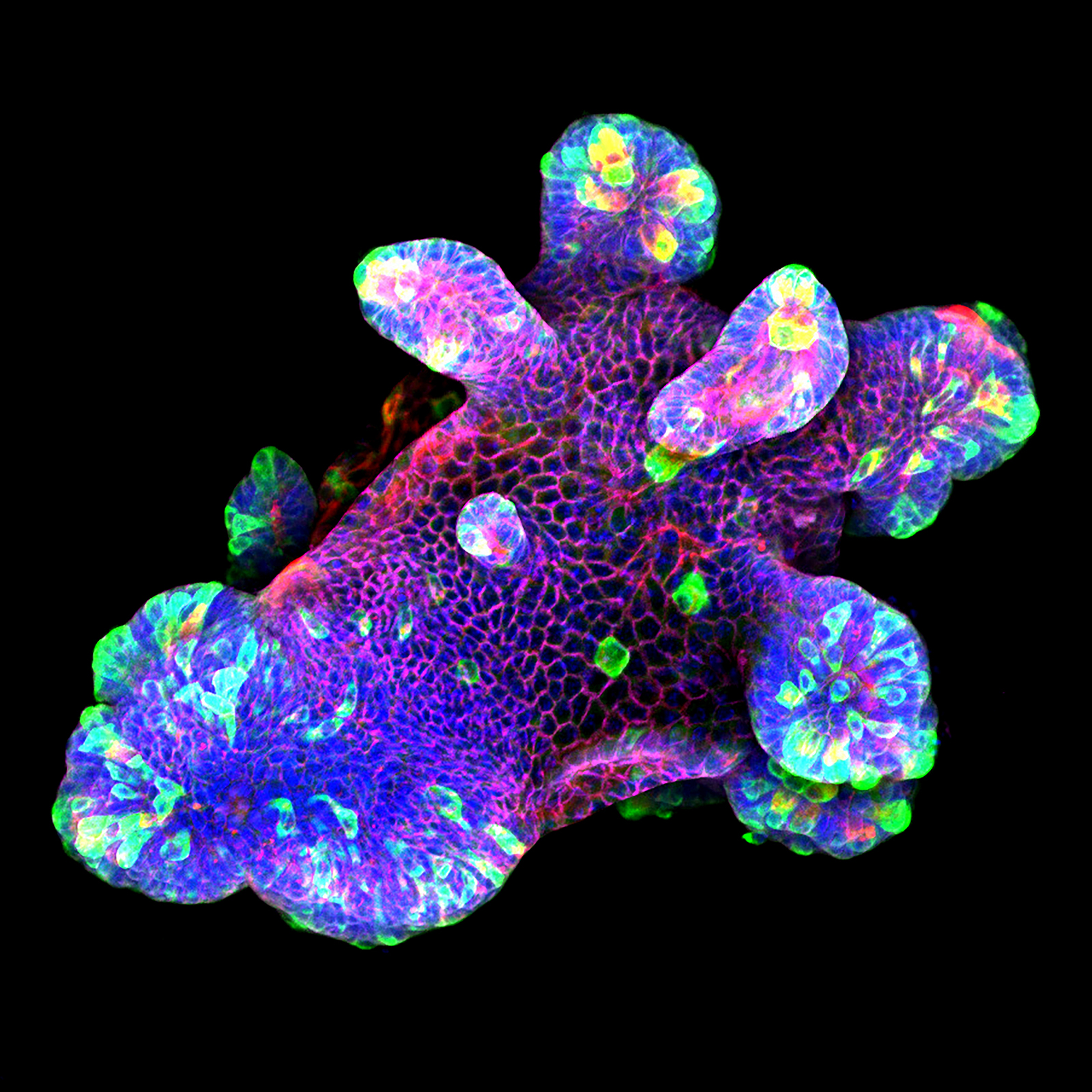
3D culture of an IL-22-treated mouse small intestine organoid grown in Matrigel® drops using an ibidi µ-Slide 8 Well. Image by Naveen Parmar, Norwegian University of Science and Technology (NTNU), Trondheim, Norway.
ibidi Solutions for Cell Cultivation in a 3D Matrix
Selected Publications for Cell Cultivation in a 3D Matrix
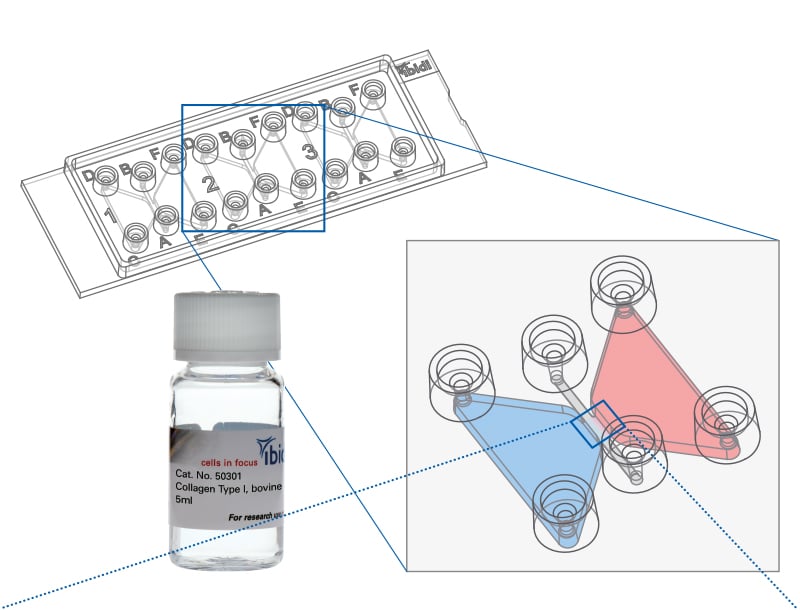
3D Chemotaxis assay with lymphoma cells in collagen matrix was done using the µ-Slide Chemotaxis.
Antonello P, Pizzagalli DU, Foglierini M, et al. ACKR3 promotes CXCL12/CXCR4-mediated cell-to-cell-induced lymphoma migration through LTB4 production. Front Immunol. 2023;13:1067885. doi:10.3389/fimmu.2022.1067885.
Read article
Murine mesenchymal-like colon cancer organoids were cultured in a 3D matrix and immunostained in the µ-Slide 15 Well 3D.
Poghosyan S, Frenkel N, Lentzas A, et al. Loss of Neuropilin-2 in Murine Mesenchymal-like Colon Cancer Organoids Causes Mesenchymal-to-Epithelial Transition and an Acquired Dependency on Insulin-Receptor Signaling and Autophagy. Cancers (Basel). 2022;14(3):671. doi:10.3390/cancers14030671.
Read article
An in vitro 3D model for cancer invasion was established in the µ-Slide 15 Well 3D.
Jouybar M, Sleeboom JJF, Vaezzadeh E, Sahlgren CM, den Toonder JMJ. An in vitro model of cancer invasion with heterogeneous ECM created with droplet microfluidics. Front Bioeng Biotechnol. 2023;11:1267021. doi:10.3389/fbioe.2023.1267021.
Read article
A liver-on-a-chip through hepatocyte 3D culture in a hydrogel was established using the µ-Slide III 3D Perfusion.
Christoffersson J, Aronsson C, Jury M, Selegård R, Aili D, Mandenius CF. Fabrication of modular hyaluronan-PEG hydrogels to support 3D cultures of hepatocytes in a perfused liver-on-a-chip device. Biofabrication. 2018;11(1):015013. doi:10.1088/1758-5090/aaf657.
Read article
The hanging drop method, a traditional approach for the generation of uniform 3D aggregates, uses gravity to promote cell aggregation at the bottom of drops. This method involves placing small droplets of cell suspension on the underside of a culture dish lid. As gravity acts, cells within these droplets migrate and aggregate at the bottom, forming a spheroid. This technique allows for precise control over the initial cell number in each droplet, leading to uniform spheroid size, an essential factor for consistent experimental results.
One of the key advantages of the hanging drop method is its simplicity and cost-effectiveness, requiring minimal specialized equipment. Additionally, it facilitates the formation of spheroids with well-defined, symmetrical structures, which is crucial for studies involving cell-to-cell interactions and tissue morphogenesis. However, while effective for spheroid generation, it is less established for organoids and not ideal for high-resolution microscopy.
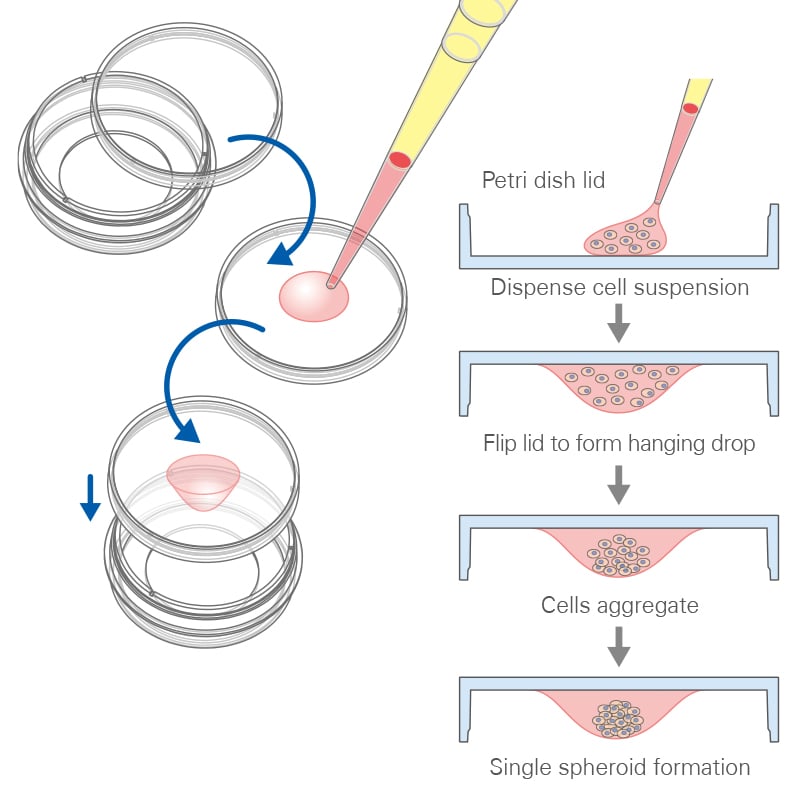
Principle of the hanging drop method.
ibidi Solution for the Hanging Drop Method
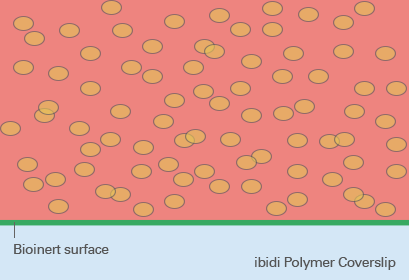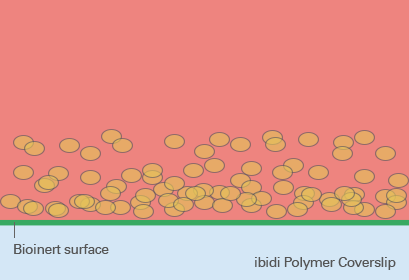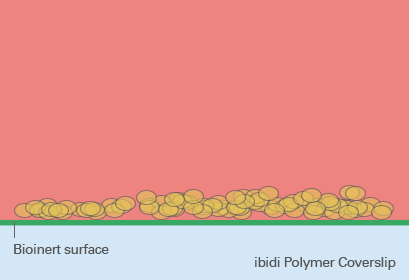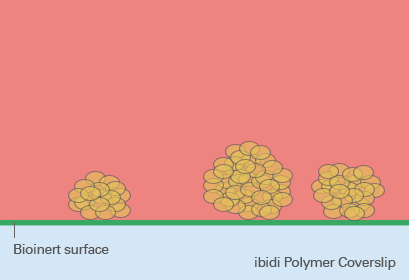
Spheroid generation on bioinert or ultra-low attachment (ULA) surfaces is a pivotal technique in cell culture, enabling the formation of three-dimensional cell aggregates. These surfaces are specially designed to prevent cell adhesion, thus encouraging cells to interact with each other rather than attaching to the substrate. This environment mimics the three-dimensional architecture of tissues in the body, making spheroids an ideal model for studying cellular behavior in a more physiologically relevant context.
The benefits of using bioinert or ULA surfaces include the ability to cultivate a wide range of cell types into spheroids, the facilitation of more natural cell-cell interactions, and the enhancement of cellular functions that may not be evident in two-dimensional cultures. However, the lack of cell attachment can make certain types of analyses more challenging, such as those requiring fixed cell positions. Furthermore, not all cell types may form spheroids equally well on these surfaces, and the homogeneity of spheroid formation can vary based on the inherent cell-cell adhesion properties of the cell type in question.
ibidi Solutions for Spheroid Aggregation on Bioinert Surfaces
Selected Publications for Spheroid Aggregation on Bioinert Surfaces
Hepatocellular carcinoma spheroids were generated and stained in the µ-Dish 35 mm, high Bioinert.
Bergamini C, Leoni I, Rizzardi N, et al. MiR-494 induces metabolic changes through G6pc targeting and modulates sorafenib response in hepatocellular carcinoma. J Exp Clin Cancer Res. 2023;42(1):145. doi:10.1186/s13046-023-02718-w.
Read article
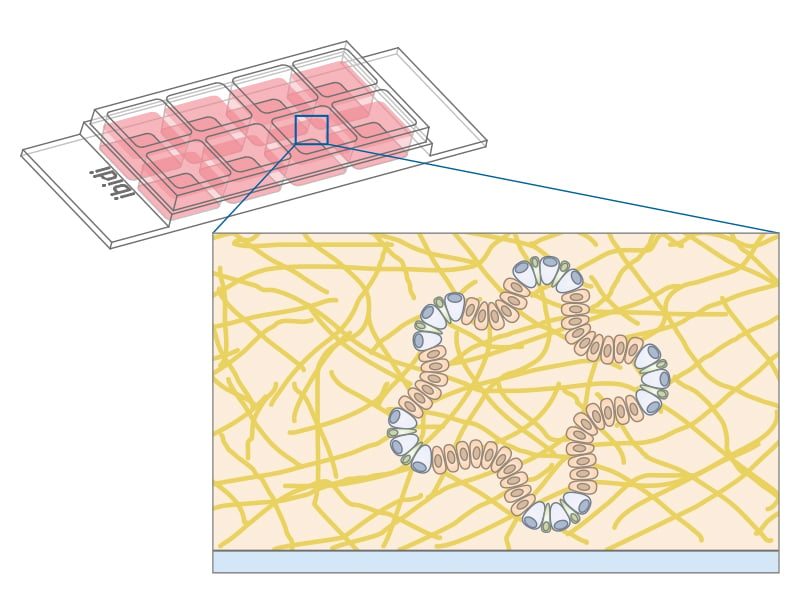
The ibidi µ-Patterning technology merges adherent spots with a Bioinert surface and thus enables precise control over the spatial and dimensional properties of the aggregate growth. This structure promotes important cell-cell interactions for the formation of 3D cell aggregates. The technology's unique capability to manipulate pattern dimensions and geometries allows for the creation of spatially defined structures. Consequently, researchers can produce spheroids with consistent size, shape, and cell distribution, enhancing uniformity and experimental reproducibility.
Combining the µ-Patterning technology with the µ-Slide 8 Well high and µ-Slide VI 0.4 enhances the efficiency of spheroid aggregation, culture assays, and imaging. These products are designed to streamline the experimental workflow by eliminating the need for cell passaging or individual well manipulation, thereby facilitating a seamless, high-throughput approach.
In particular, the µ-Slide VI 0.4 With Multi-Cell µ-Pattern allows for the simultaneous generation of multiple spheroids, with a capacity of up to 180 spheroids in one channel. This translates to the potential of producing approximately 1080 spheroids per µ-Slide. This feature not only increases throughput but also ensures consistency and precision in spheroid formation, crucial for advanced cell culture experiments.
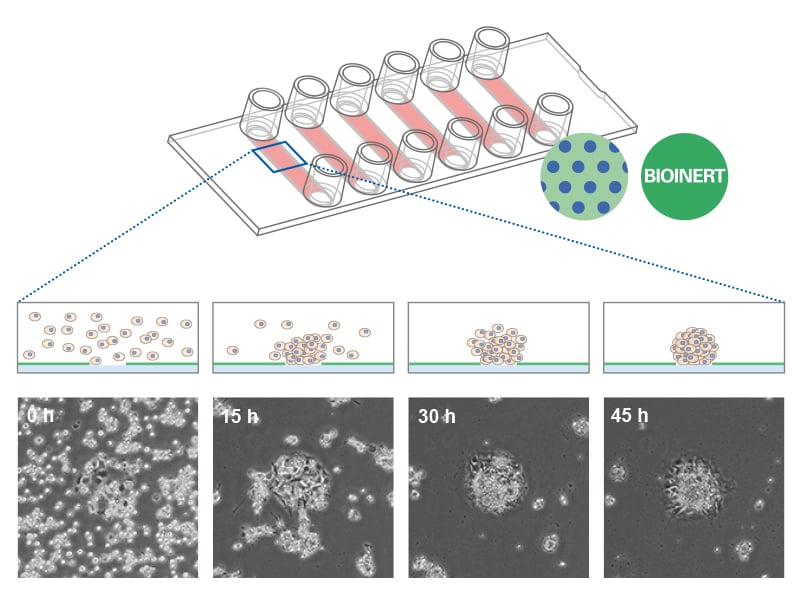
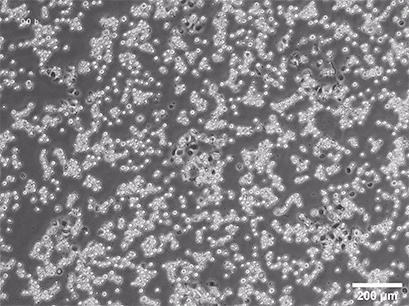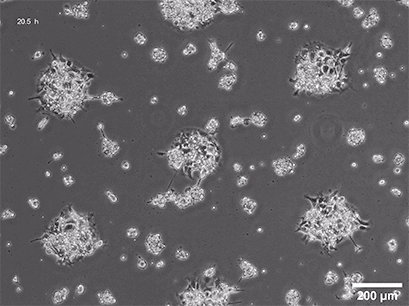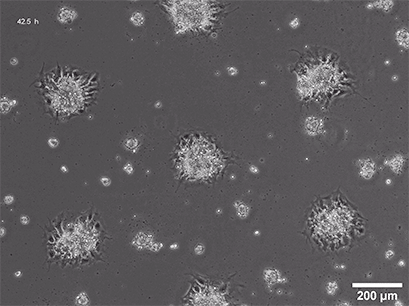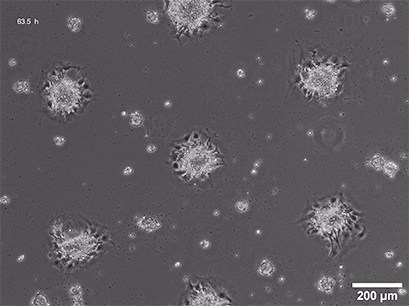
Spheroid formation of NIH-3T3 cells (murine embryo fibroblasts) on 200 µm adhesion spots using ibidi µ-Patterning. Spheroid generation was documented for 64 hours. Phase contrast live cell imaging, 4x objective lens.
ibidi Solutions for Spheroid Aggregation Using µ-Patterning
Interested in Spheroid Aggregation on a
µ-Pattern Under Flow?
This facilitates continuous media exchange and long-term cultivation, essential for creating physiological conditions that closely mimic the in vivo environment.
Read on and have a look at methods for Advanced Cultivation of 3D Cell Models or Experimental Applications.